Nasal Esketamine (Spravato) and Breastfeeding
Summary
Esketamine can be part of the psychiatric management of treatment-resistant depression1,2 and its compatibility with breastfeeding is currently unknown.2,3 Due to its chemical properties, esketamine likely penetrates breastmilk to a low degree.3 More information about the pharmacokinetics of this drug may help a breastfeeding mother make an informed decision regarding lactation and concurrent use of intranasal esketamine.
Depression and Esketamine
Like its chemical parent ketamine, esketamine acts on NMDA receptors within the central nervous system. Intranasal esketamine (S-ketamine, SPRAVATO) is used as adjunct therapy for treatment-resistant depression and major depressive disorder with suicidality.1,2,4 Doses and schedules vary by indication and phase of treatment. Doses can be as high as 84 mg twice per week or as low as 56 mg every two weeks for maintenance therapy.1,2,5 Specifics can be found on the Spravato website.
In adults, intranasal esketamine can cause hypertension, dizziness, anxiety, lethargy, vertigo, sedation, dissociative reactions, nausea, and vomiting.2 For these reasons, patients are required to be monitored by medical personnel for at least two hours following each dose. After patients return home, they are asked to avoid activities that require complete alertness for a day after treatment. Even without considering drug transfer into breastmilk, a mother’s capacity to care for her child will likely be impacted during this period of time.
Breastfeeding and esketamine
Limited observations and evidence
Per the product manufacturer, women are advised not to breastfeed if using esketamine.6 However, this is a common response by pharmaceutical companies in response to a lack of available human data. There are no reports of adverse effects in breastfed infants, but we don’t know how many infants have previously been exposed to the drug through breastmilk.
The manufacturer (Janssen) conducted animal studies that showed sensorimotor delays in offspring born from animals given intranasal esketamine during gestation and lactation. Because of the prolonged exposure, it is difficult to assess whether the results are correlated to exposure during gestational development, lactation, or both. Animal studies conducted on the offspring of lactating animal-subjects stated that their young (not given esketamine) had detectable plasma concentrations of the drug when drinking milk from the mother taking intranasal esketamine.2 Other than detectable esketamine in the blood, no other details were listed regarding the effects of esketamine on this group of animal subjects.2 However, a report by another manufacturer (Pfizer) states that injectable esketamine “is excreted into breast milk, but an effect on the child seems unlikely when using therapeutic doses.”7 The research referenced in this document has not been published, and our requests for information thus far have been disregarded. There have been studies of the impact of ketamine use during cesarean sections, but milk supply in these studies had not yet been established, therefore milk transfer could not be evaluated.
Without a clear answer from research, we have to turn to our understanding of pharmacokinetics to estimate an infant’s exposure to esketamine through breastmilk. To begin this evaluation, first we must understand the drug in the mother.
Characteristics of Esketamine
Estimates of Esketamine Transfer to Milk
Ketamine is a 1:1 racemic mixture of r-ketamine and s-ketamine. It is a potent NMDA receptor antagonist, but also has antidepressant and pain modifying effects through unknown mechanisms.4,6 The antidepressant effects long outlast the duration that the drug is in the body.8 Different formulations of ketamine are used intravenously as anesthetics for pain-relief and sedation.9,10 Esketamine is the purified s-ketamine enantiomer and is only used intranasally. When there is limited information on esketamine, data for ketamine can be used as a fair surrogate.
Intranasal formulations of esketamine have a relatively high bioavailability, meaning that a large proportion of the drug enters the bloodstream to produce its effects. Nearly half (48%) of the intranasal dose enters the circulation, then the remaining dose is swallowed. Only 19% of the swallowed esketamine reaches systemic circulation, most is sequestered in the liver.11 By this logic, 60% of the dose reaches maternal circulation. The low oral bioavailability is a further benefit to the baby, as less than 20% of the dose they receive via milk will be absorbed. Ultimately, little drug will be absorbed by the infant compared to the mother’s dose.
Experience with other drugs suggest that concentrations of drugs in milk are directly related to plasma concentrations. A small study of healthy volunteers measured the concentration of ketamine in the blood. Participants who took 25 mg of intranasal racemic ketamine had undetectable plasma concentrations 8 hours after administration of the drug.6 These doses were less than half of a therapeutic dose.2,10 However, the manufacturer reports that drug exposure in the plasma is fairly dose-proportional.2 If ketamine is not in the plasma after 8 hours, soon afterward it will also not be in the milk.
Esketamine has no known relative infant dose (RID), though more trials testing its levels in milk may soon be available.12 Because of its relatively low molecular weight and ability to penetrate the central nervous system, it is suggested that esketamine may be present in breast milk.3 However, there are many more considerations to determine exposure through milk and clinical risk for an infant.
The volume of distribution (Vd) is the way we estimate where the drugs end up in the body. Drugs with large Vd, generally end up outside the blood compartment such as in muscle or fat. Drugs that remain in the blood have a low volume of distribution, similar to the volume of blood plasma in the body (~5L). The Vd of esketamine is reported as 709 L (9.8 L/kg for a 72kg patient), indicating that it redistributes outside of the plasma extensively.2 As mentioned, drugs are only capable of transferring into the milk compartment if they are located in blood compartment. Therefore, esketamine’s extensive volume of distribution is expected to significantly reduce the amount of drug available for transfer into milk.
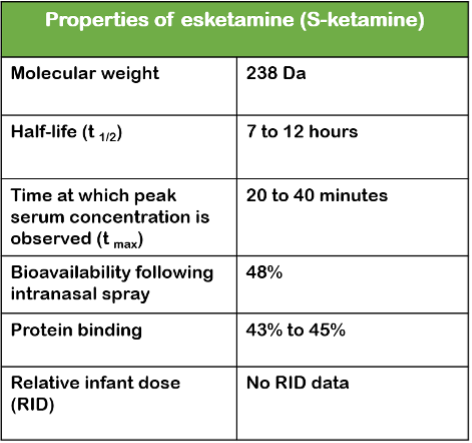
Esketamine is metabolized by the liver and does not accumulate in the blood. It has an active metabolite (noresketamine), but it is not as potent and is less concentrated in the CNS. Esketamine has a biphasic elimination curve—it is rapidly metabolized for the first 3 hours, then more slowly for a terminal half-life of 7-12 hours.2
The following table shows the properties of Spravato®.2
Minimizing infant exposure
When esketamine is administered intranasally it takes 20 to 40 minutes to reach a peak concentration in the blood (and then in milk). After reaching its peak, the concentration in the blood then decreases rapidly as esketamine is metabolized, excreted or redistributed to remote compartments in the body.2,10
Interrupting breastfeeding on days of treatment may not be suitable for many moms, particularly with a young infant. Waiting for “total” elimination may not work for mothers, especially during their first few weeks of treatment. Withholding breastfeeding for 8 hours after a treatment session would reduce infant exposure to the drug significantly.
Monitoring a breastfed infant
Using medications in breastfeeding moms is always a case of risk vs benefit. In the case of refractory depression, the risk to the infant from maternal depression is significant. Further, the benefit of breastfeeding is enormous. Infants are in a critical stage of neurocognitive development, and esketamine is a potent medication that might affect the nervous system.2 However, the benefits of breastfeeding3 may outweigh the risks in certain cases. A careful evaluation from both the mother and infant’s medical team are necessary.
If a mother chooses to breastfeed, carefully monitor symptomatic changes and observe the breastfed infant before, during, and after each feeding to note any differences.
Breastfed infants should be monitored closely for signs of sedation—if the baby is difficult to wake or has slowed breathing.2,3 Neurotoxicity and potential injury to the infant’s nervous system should be monitored as well by establishing a baseline with the infant’s healthcare provider. Infants should be observed and monitored for cognitive delays and sensorimotor changes.2,4
Neonates with any conditions effecting the liver could be more susceptible to the potential effects of esketamine from breast milk as this drug is largely metabolized in the liver and any damage to the liver might affect elimination of this drug.2
Effects on lactation
There is no evidence that esketamine effects milk production. A randomized double-blind study in 56 women showed that patients who received pain medications plus IV esketamine for caesarian delivery were able to breastfeed for the same amount of time as those who received pain medications plus placebo (no esketamine), with no reported effects on lactation.4
Current research
There are currently studies underway looking at the transfer and safety of esketamine and ketamine for a breastfeeding dyad. Results are pending, but no untoward effects in infants have been noted thus far. If you take these products and would like to donate your milk for research, please contact us.
Conclusion
Due to limited information concerning the transfer of esketamine into milk and its adverse effects on breastfed infants, it is not possible to conclude that it is safe to use in breastfeeding mothers. Esketamine likely transfers into milk to some degree, as is normal for any drug. Close monitoring of the infant should be prioritized if planning to breastfeed while taking intranasal esketamine as a treatment for depression. Following safety recommendations and using the evidence provided above, consider the benefits and risks for both mother and infant before making an informed decision. A brief waiting period of 8 hours following the dose of ketamine might significantly reduce any exposure in the infant.
Kaytlin Krutsch, PharmD, MBA, BCPS
Thomas W Hale, PhD, RPh
Adapted by an article written by Clarissa A Ramirez, MSIV, MBA
References:
1. Serafini G, Howland RH, Rovedi F, Girardi P, Amore M. The role of ketamine in treatment-resistant depression: a systematic review. Current neuropharmacology. 2014;12(5):444-461.
2. Janssen. Product Monograph: Esketamine Nasal Spray (Spravato Product Monograph). In:2020.
3. Hale T. Medications and Mothers’ Milk, 2021: A Manual of Lactational Pharmacology. Springer; 2020.
4. Esketamine. In: Drugs and Lactation Database (LactMed) [Internet]. National Library of Medicine (US); 2019.
5. Quintana DS, Guastella AJ, Westlye LT, Andreassen OA. The promise and pitfalls of intranasally administering psychopharmacological agents for the treatment of psychiatric disorders. Molecular Psychiatry. 2016;21(1):29-38.
6. Ketamine. In: Drugs and Lactation Database (LactMed) [Internet]. National Library of Medicine (US); 2021.
7. Pfizer. Annex to PSUR: Core Safety Profile s-Ketamine (ketamine hydrochloride) solution for injection. In:2009.
8. Sleigh J, Harvey M, Voss L, Denny B. Ketamine – More mechanisms of action than just NMDA blockade. Trends in Anaesthesia and Critical Care. 2014;4(2):76-81.
9. American Society of Anesthesiologists. Statement on Resuming Breastfeeding after Anesthesia. https://www.asahq.org/standards-and-guidelines/statement-on-resuming-breastfeeding-after-anesthesia. Published 2019. Updated 2019/10/23. Accessed 2021/7/29.
10. Yanagihara Y, Ohtani M, Kariya S, et al. Plasma concentration profiles of ketamine and norketamine after administration of various ketamine preparations to healthy Japanese volunteers. Biopharmaceutics & Drug Disposition. 2003;24(1):37-43.
11. Perez-Ruixo C, Rossenu S, Zannikos P, et al. Population Pharmacokinetics of Esketamine Nasal Spray and its Metabolite Noresketamine in Healthy Subjects and Patients with Treatment-Resistant Depression. Clin Pharmacokinet. 2021;60(4):501-516.
12. ClinicalTrials.gov. The Pharmacodynamics of Ketamine in the Breast Milk of Lactating Women. https://clinicaltrials.gov/ct2/show/NCT04285684. Published 2021. Updated 2021/07/29/. Accessed 2021/7/29.