Plain Language Summary
Domperidone is a medication approved in some countries to increase gastric motility and reduce nausea and vomiting. The chemical structure of domperidone is similar to antipsychotic medications. In recent decades, domperidone has gained popularity in the breastfeeding community and has been used off-label to treat low milk supply. Domperidone’s major drawbacks are rare, but severe, including heart complications (cardiac arrhythmias due to QT-prolongation), weight gain, and anxiety or depression when it is discontinued. The benefit of domperidone is highly individual. Average increases in daily milk volume are around 1.7 ounces (60 mL), which can be significant for premature infants, but less so for babies as they grow older. Currently domperidone is banned in the United States (US) and its consumption may complicate receiving healthcare in the US.
Importance of breastfeeding and Low Milk Supply
Breastfeeding provides unique health benefits for at least the first six months of an infant’s life and can promote a strong emotional bond between parents and infants. Breastfed babies tend to have fewer allergies and infections, reduced risks of asthma, and lower risk of obesity and diabetes in adulthood1. Breastfeeding also has maternal benefits such as promoting faster weight loss, lowering the risk of some cancers, and reducing the risk of certain chronic conditions such as diabetes2.
Up to 83% of mothers initiate breastfeeding, but by 6 months, only 25% of US mothers are exclusively breastfeeding; 60% of the women who stop breastfeeding in the first 6 months indicate that cessation occurred earlier than they had planned3. One of the main reported reasons why mothers stop breastfeeding is due to the concern that they do not have enough milk to feed their baby (low milk supply)3 4.
Domperidone and prolactin
There are many maternal factors that can contribute to low milk supply. For the purpose of this article, we will briefly discuss hormones that impact lactation and why domperidone has been prescribed to help with low milk supply. Domperidone is theorized to block dopamine receptors in the pituitary and other areas of the body. By blocking dopamine, domperidone would trigger the release of prolactin, a hormone that stimulates milk production. It is important to understand that there are several hormones involved in milk production other than prolactin. Other important hormones include progesterone, cortisol, thyroid stimulating hormone, and oxytocin5. The combination of hormones and a consistent removal of milk from the breast work together to create a sufficient milk supply5. Therefore, increasing prolactin alone does not always result in increased milk supply. The multi-faceted requirements for sufficient milk supply explain why women have varying responses to domperidone. Some women see a drastic increase in milk volume (up to 94 mL/day or 3 oz) while other women see no change in milk supply 6. We do not have any evidence-based answers to who would benefit from domperidone.
Domperidone use in the USA
Domperidone has not been approved to treat low milk supply anywhere in the world. It is used “off label” in some countries, like Canada. The US Food and Drug Administration (FDA) has not approved domperidone for any use in the USA. While there many technical arguments made against this fact, the short explanation is that the FDA explicitly warns against using or illegally importing domperidone in breastfeeding mothers. Compounding pharmacies are also not allowed to sell domperidone in the US. Domperidone is only legally available in the US through regulated research programs for gastrointestinal disorders7.
Despite this standing, US parents appear to be purchasing domperidone for use as a galactagogue. While parents make these decisions with positive intent, when related medical issues arise, addressing appropriate healthcare outside of lactation becomes complicated. Mothers may have difficulty receiving informed, timely medical care due to lack of provider familiarity with domperidone or its potential complications8,9. Parents may also be reluctant to report domperidone use in the US, leaving healthcare providers with incomplete histories and inaccurate medical diagnoses.9 Domperidone has many drug-drug interactions (more on this below), and may play a role in misdiagnoses. Always report domperidone use to medical providers (especially prescribers and pharmacists) as a vital piece of shared-decision making, keeping mothers safe.
Does domperidone transfer into milk affect breastfed infants?
We are happy to report that there is very little risk of domperidone having any direct impact on breastfed infant10. Even at high doses of 160 mg per day, the transfer of domperidone into breast milk was negligible with a relative infant dose (RID) of 0.05%10. Therefore, absolute infant doses via milk are miniscule. However, mothers experiencing psychiatric withdrawal reported significant strain on the maternal-infant bond during this time11.
Cardiac effects of domperidone
Although lactating women are often generalized as having a low cardiac risk, this generalization is not accurate for all women. A standard medical history, current medication list, and electrocardiogram (ECG or EKG) can easily identify most women at high risk of domperidone’s known cardiac effects.
Domperidone can trigger abnormal and life-threatening arrhythmias (when the heart beat goes astray). Specifically, domperidone lengthens the QT-interval, which can disturb the heart’s electrical conduction system12,13. QT-intervals are a measurement of the electrical conductivity of the heart, easily calculated via EKG. Drugs that prolong the QT interval (including domperidone) can trigger ventricular arrhythmia, which make the heart less effective at pumping blood, or in rare cases can cause sudden cardiac death12,14. Factors that can increase the risk of domperidone’s QT prolonging effects include higher doses, history of ventricular arrhythmias, known prolonged QT intervals, obesity, and taking products that interact with domperidone15. Though not recommended, breastfeeding mothers are often prescribed domperidone for extended periods and at doses higher than 30 mg/day. These factors carry a higher risk for cardiac arrythmias and complication 16.
Numerous other prescription drugs can prolong the QT-interval, including some antibiotics (e.g., ciprofloxacin, azithromycin, etc.), antidepressants (e.g., escitalopram), antipsychotics (e.g., quetiapine) and many others 17. Many patients are not routinely screened with an EKG or to advised to avoid the use of concomitant QT-prolonging drugs. Domperidone also has significant drug-drug interactions, especially with other medications that are CYP3A4 inhibitors 12. CYP3A4 is a group of liver enzymes that help metabolize drugs and food in the body 18. Certain drugs, like domperidone, have the effect of inhibiting CYP3A4, preventing drug clearance, which increases the level of the drug in the body making it more toxic 12. Many medications and some foods are CYP3A4 inhibitors and should not be used at the same time as domperidone. These include some medications that are frequently used by lactating women such as fluconazole, diltiazem, verapamil, ritonavir, erythromycin, and clarithromycin 12. Foods like grapefruit juice are CYP3A4 inhibitors and should be avoided while taking domperidone 19. Herbal supplements like St. John’s wort also interact with CYP3A420.
Neuropsychiatric effects of domperidone
Domperidone is usually categorized as a dopamine antagonist. It not typically associated with similar drugs in its class such as antipsychotics. This is primarily due to the belief that it does not cross the blood-brain barrier. Notably, antipsychotics are infamous for their array of side effects, including weight gain, movement disorders, anxiety, fatigue, and depression. Interestingly, these concerns mirror side effects of mothers prescribed high doses of domperidone when it is discontinued. Severe withdrawal effects reported by some breastfeeding mothers when trying to discontinue domperidone have included intense anxiety, panic, dizziness and problems with balance, dry eyes/mouth, gastric problems, akathisia (restlessness), confusion, insomnia, and, in some severe cases, symptoms of psychosis 9,21,22. The accumulation of patient reports suggests that the drug may cross the blood-brain barrier more substantially than previously assumed. In August 2023, Health Canada reported association between psychiatric adverse events and the recent cessation of domperidone in lactating mothers.
How to safely stop using domperidone
Domperidone at any dose should not be stopped abruptly. Normal doses are 30-60 mg daily. We do not recommend ever exceeding 60 mg daily, and prefer doses over 30 mg not be used due to the risks discussed above. When discontinued, it should be guided by a written tapering plan that considers the baseline domperidone dose and patient ability to tolerate the downward titration. A common titration regimen is to, once per week, decrease the daily dose by 10 mg per day. For example, if the baseline dose is 30 mg/day (3 tablets), eliminate one 10 mg tablet for a revised total dose of 20 mg/day and stay at this dose for one week, or every two weeks if necessary 9,23.
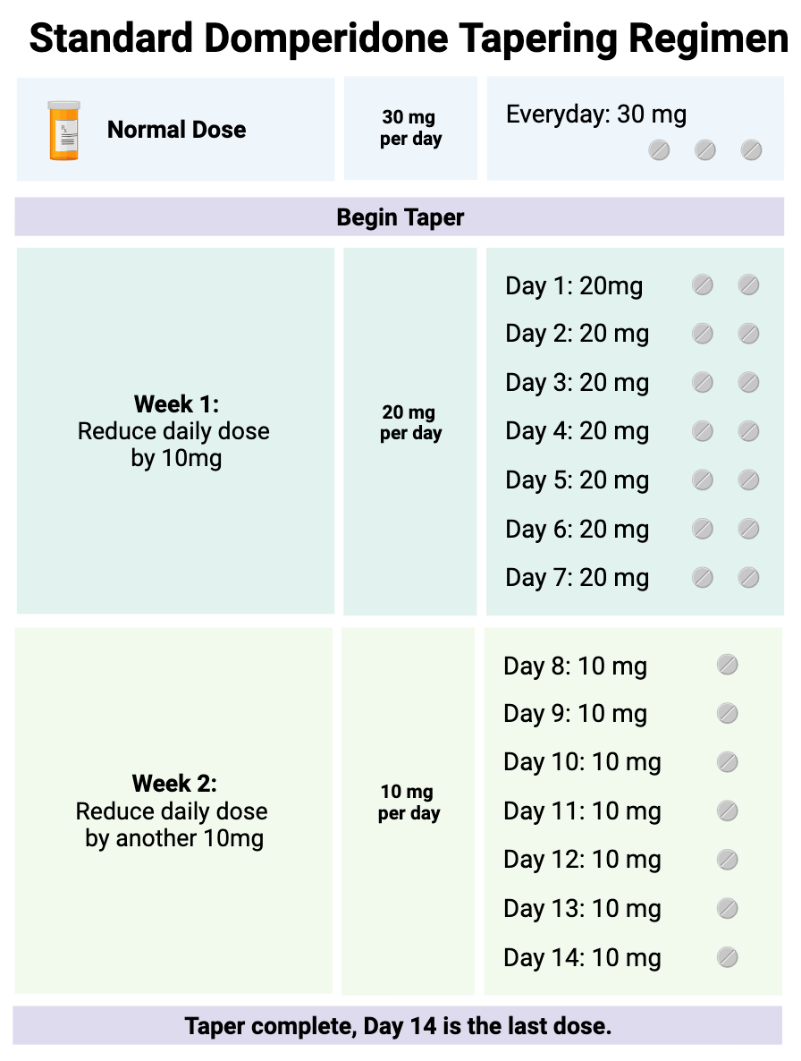
If withdrawal symptoms occur, consider extending the downward titration interval (days between dose reductions) or a less intensive dose reduction in domperidone dose.
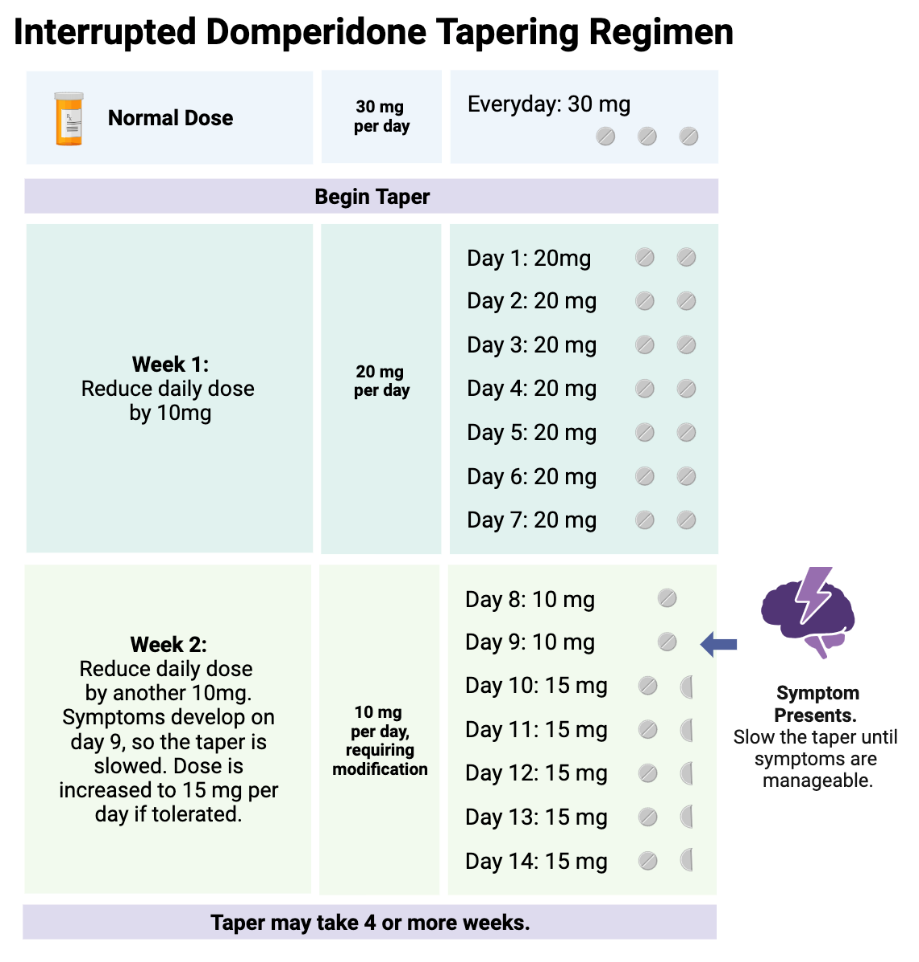
Unfortunately, there is no one size fits all regarding domperidone downward titrations; much of it depends on patient tolerance. We have seen some patients be successful titrating in weekly increments of 2.5mg or less. Hyperbolic strategies may be helpful, and usually require the assistance of psychiatric medical professionals 24. With these small doses, a pharmacist may be required to improvise dosage forms to accurately divide the dose (domperidone comes in 10 mg tabs; splitting into quarters or smaller is challenging). If a mother is taking large doses, the tapering schedule may last a year or longer to adequately manage symptoms of withdrawal.
Does domperidone increase milk supply
The data available to definitively say to what degree domperidone increases milk supply, or who it will work for, is significantly lacking. There have been multiple meta-analyses of domperidone use as a galactagogue in mothers of preterm infants that demonstrated increased milk production. Overall, results were statistically similar to placebos and mothers who adjusted their breastfeeding practices 25-28. However, statistical, and clinical definitions of significance are often at odds. Clinically “significant” increases in milk volume may be defined differently at different stages of infants’ lives and health, making a determination of what is a “significant” increase in milk volume highly individualized.
Conclusion
Due to conflicting information from trials with domperidone and the inherent safety risks, domperidone for breastfeeding mothers with insufficient milk supply must always be considered only with careful consideration of the medical team through shared decision making. Breastfeeding mothers in the US with desires to enhance milk supply should first seek expert advice from a certified lactation consultant or their medical doctor to identify proper breastfeeding techniques and rule out physiological reasons for low milk supply. Providers should diligently investigate individualized patient risk, be vigilant for potential symptoms of domperidone withdrawals, specifically and nonjudgmentally ask about domperidone use in lactating patients, and offer a titration plan with close patient monitoring and support when the patient is ready to stop taking domperidone. If you are a mother who chooses to take domperidone for low milk supply, we encourage you to be honest with your provider despite your location and the legality of how you obtained domperidone to ensure that you receive appropriate medical treatment.
Nichole Campbell, MSN, APRN, NP-C
Kaytlin Krutsch, PharmD, BCPS
Developed from a previous draft written by Janet Currie, PhD.
REFERENCES
1. Szugye H, Murra A, Lam SK. A new policy update on breastfeeding: What all clinicians need to know. Cleveland Clinic Journal of Medicine. 2023;90(8):469-473. doi:10.3949/ccjm.90a.22099
2. Sattari M, Serwint JR, Levine DM. Maternal Implications of Breastfeeding: A Review for the Internist. Am J Med. 2019/08/01/ 2019;132(8):912–920. doi:10.1016/j.amjmed.2019.02.021
3. Facts About Nationwide Breastfeeding Goals. 2023.
4. Odom EC, Li R, Scanlon KS, Perrine CG, Grummer-Strawn L. Reasons for earlier than desired cessation of breastfeeding. Pediatrics. Mar 2013;131(3):e726-32. doi:10.1542/peds.2012-1295
5. Lee S, Kelleher SL. Biological underpinnings of breastfeeding challenges: the role of genetics, diet, and environment on lactation physiology. American Journal of Physiology - Endocrinology and Metabolism. 2016/08/08/ 2016;311(2):E405. doi:10.1152/ajpendo.00495.2015
6. Domperidone. Drugs and Lactation Database (LactMed®). National Institute of Child Health and Human Development; 2006.
7. FDA. Information about Domperidone. 2023.
8. McBride GM, Stevenson R, Zizzo G, et al. Women’s experiences with using domperidone as a galactagogue to increase breast milk supply: an Australian cross-sectional survey. Int Breastfeed J. 2023/12// 2023;18(1):1–9. doi:10.1186/s13006-023-00541-9
9. Majdinasab E, Haque S, Stark A, Krutsch K, Hale TW. Psychiatric Manifestations of Withdrawal Following Domperidone Used as a Galactagogue. Breastfeeding Medicine. 2022/12/05/ 2022;17(12):1018–1024. doi:10.1089/bfm.2022.0190
10. Krutsch K, Datta P. The Transfer of Domperidone into Human Milk Remains Low at High Doses. Breastfeed Med. Jul 2023;18(7):555-556. doi:10.1089/bfm.2023.0108
11. Majdinasab E, Haque S, Stark A, Krutsch K, Hale TW. Psychiatric Manifestations of Withdrawal Following Domperidone Used as a Galactagogue. Breastfeed Med. Dec 2022;17(12):1018-1024. doi:10.1089/bfm.2022.0190
12. Domperidone (Lexi-Drugs) - Lexicomp. 2024.
13. Summary Safety Review - DOMPERIDONE - Serious abnormal heart rhythms and sudden death (cardiac arrest) - Drug and Health Products Portal. 2024.
14. Kalwar A, Mangi RQ, Rehman JU, et al. Lack of knowledge regarding the misuse of domperidone in Pakistan and its serious consequences: short communication. Ann Med Surg (Lond). 2023/04/27/ 2023;85(6):3239–3240. doi:10.1097/ms9.0000000000000723
15. Brodribb W. ABM Clinical Protocol #9: Use of Galactagogues in Initiating or Augmenting Maternal Milk Production, Second Revision 2018. Breastfeeding (Ninth Edition). Elsevier; 2022:853–861.
16. Ou LB, Moriello C, Douros A, Filion KB. Domperidone and the risks of sudden cardiac death and ventricular arrhythmia: A systematic review and meta-analysis of observational studies. Br J Clin Pharmacol. 2021/10/01/ 2021;87(10):3649–3658. doi:10.1111/bcp.14737
17. LexiComp. Drug-Induced Prolongation of the QT Interval and Torsades de Pointes (Lexi-Drugs) - Lexicomp. Updated 2024/01/03/. https://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/5712802?cesid=1KaNrrzf4TS&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dqt%2Bprolongation%2Bdrugs%26t%3Dname%26acs%3Dtrue%26acq%3Dqt%2Bprolong
18. CYP3A4 - EBSCO. 2024.
19. Drug Metabolism - The Importance of Cytochrome P450 3A4. 2023.
20. Roby CA, Anderson GD, Kantor E, Dryer DA, Burstein AH. St John's Wort: effect on CYP3A4 activity. Clin Pharmacol Ther. May 2000;67(5):451-7. doi:10.1067/mcp.2000.106793
21. Cerovecki A, Musil R, Klimke A, et al. Withdrawal Symptoms and Rebound Syndromes Associated with Switching and Discontinuing Atypical Antipsychotics: Theoretical Background and Practical Recommendations. CNS Drugs. 2013/07/01 2013;27(7):545-572. doi:10.1007/s40263-013-0079-5
22. Doyle M, Grossman M. Case report: domperidone use as a galactagogue resulting in withdrawal symptoms upon discontinuation. Archives of Women's Mental Health. 2018/08/01 2018;21(4):461-463. doi:10.1007/s00737-017-0796-8
23. Seeman P. Yes, breast is best, but taper domperidone when stopping. Br J Gen Pract. 2023/12/28/ 2023;
24. Korchia T, Abdelhafez H, Bretelle A, Joober R, Palaniyappan L. Collaborative discontinuation of antipsychotics after the first episode of psychosis. Journal of psychiatry & neuroscience : JPN. Canada: Canadian Medical Association; 2023. p. E265-E266.
25. Shen Q, Khan KS, Du MC, Du WW, Ouyang YQ. Efficacy and Safety of Domperidone and Metoclopramide in Breastfeeding: A Systematic Review and Meta-Analysis. Breastfeed Med. Jul 2021;16(7):516-529. doi:10.1089/bfm.2020.0360
26. Taylor A, Logan G, Twells L, Newhook LA. Human Milk Expression After Domperidone Treatment in Postpartum Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Hum Lact. Aug 2019;35(3):501-509. doi:10.1177/0890334418812069
27. Grzeskowiak LE, Smithers LG, Amir LH, Grivell RM. Domperidone for increasing breast milk volume in mothers expressing breast milk for their preterm infants: a systematic review and meta-analysis. Bjog. Oct 2018;125(11):1371-1378. doi:10.1111/1471-0528.15177
28. Knoppert DC, Page A, Warren J, et al. The effect of two different domperidone doses on maternal milk production. J Hum Lact. Feb 2013;29(1):38-44. doi:10.1177/0890334412438961